More than two years since COVID-19 began spreading across the world, we continue to feel its impact. Clinical trials, which were suspended during the pandemic and have since restarted, have created a research backlog. Sponsors and sites are subsequently struggling to cope with such high enrollment demands. In a recent webinar, our Founder and Chief Executive Officer, Kate Shaw, was joined by Whitney Taynton, our US-based Business Unit Head, and Jane Thurston, Senior Patient Recruitment Lead for Parexel, to discuss strategies for accelerating patient recruitment in clinical trials post-COVID-19 that will satisfy both sites and sponsors.
Clinical trial patient recruitment has always been challenging. Even before the COVID-19 pandemic, it was estimated that around four in five studies fail to meet their recruitment deadlines. But in the last two years, the landscape of clinical trials and patient recruitment and engagement has shifted. This brings new challenges but also new opportunities to approach things differently.
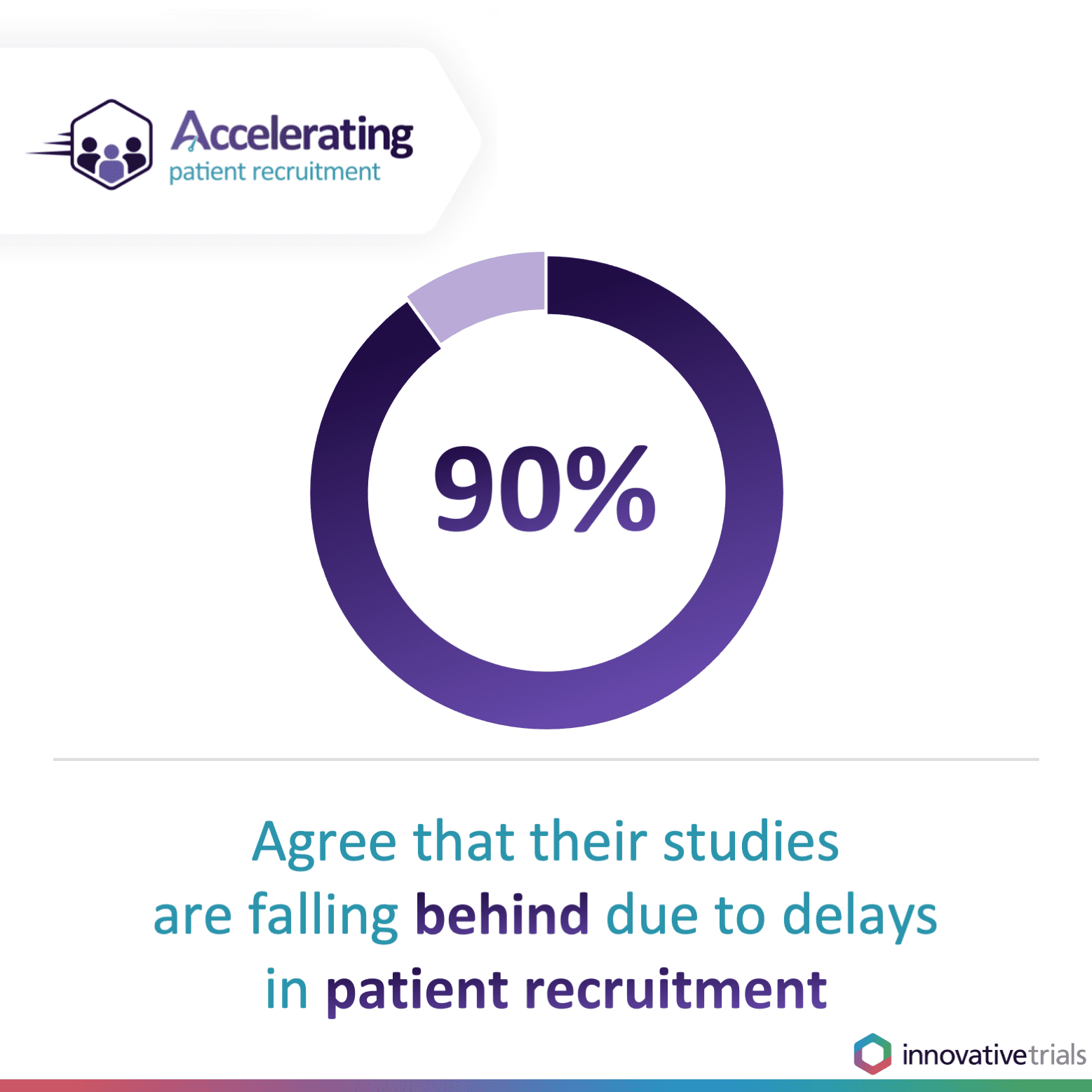
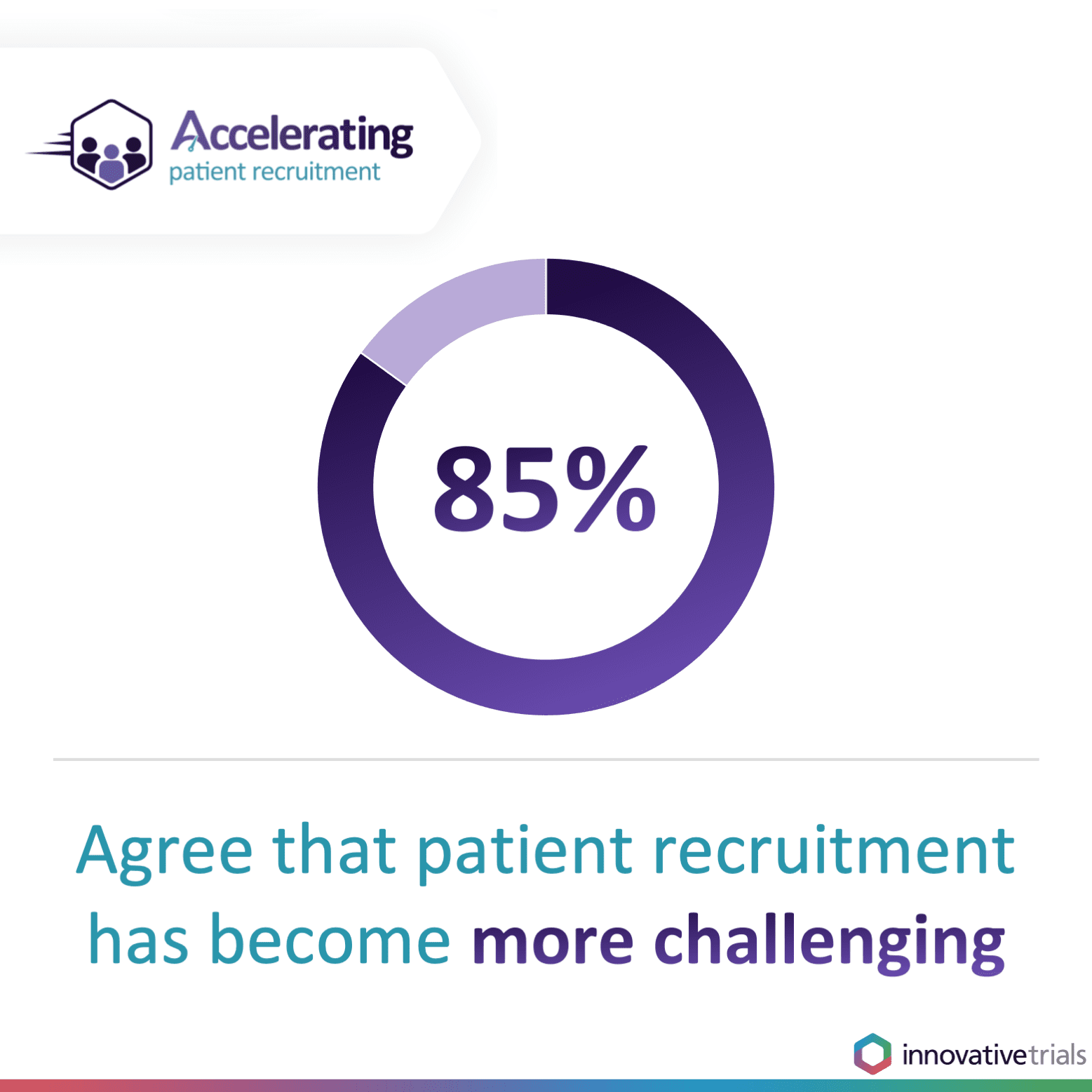
The pandemic forced the temporary suspension of non-COVID-related clinical trials as hospital units and healthcare staff focused on treating those most seriously ill with the virus. But now, as sponsors make up for lost time by restarting trials and commissioning even more, there is unprecedented demand to accelerate clinical trial patient recruitment to keep the drug development pipeline moving. Of those who attended our webinar, more than four in five (85 per cent) agreed that clinical trial patient recruitment has become more challenging since COVID-19 and nine in 10 (90 per cent) reported that their current studies are falling behind due to delays in patient recruitment.Difficulties finding adequate numbers of eligible patients for clinical trials is one of the key reasons why clinical trials overrun. This drives up costs for sponsors, puts pressure on sites and potentially delays or halts access to vital treatments. It is why accelerating clinical trial patient recruitment is critical.
Patient recruitment challenges post-COVID-19
COVID-19 has altered the dynamics of clinical trial patient recruitment, particularly as sponsors look to rescue their trials in different therapeutic areas due to the delays imposed by the pandemic. “Patient recruitment in 2022 is not what it was several years ago,” noted Whitney Taynton. “I think it’s going to take years to rebound from the pandemic and the issues that it brought to the surface.” These challenges include those already present within patient recruitment practice, such as the need to include more people of color, as well as new challenges such as elements of decentralized trials.
Patient diversity
At Innovative Trials, we have long been vocal in emphasizing the need for greater patient diversity in clinical trials. Inclusive trials are the only way to gather the evidence needed to understand how treatments affect different patient populations and confirm optimum dosing. The COVID-19 vaccine trials have been some of the most diverse in terms of including different patient populations, particularly those from racially and ethnically diverse communities. We are seeing more sponsors acknowledging the need for more diversity in trials, but this is not a simple request to fulfill.
“The struggle is that so many patients of color are fearful,“ Jane Thurston said. “…they don’t want to be made to be guinea pigs or they’re afraid that because they’re of color they’ll be the ones that get the placebo versus people who are Caucasians. [There was] a higher percentage of patients of color dying from Covid because they were afraid to get vaccinated, and that stems from what has occurred historically in the past to patients of color in clinical trials. I’m sure that most of the people who are joining on this webinar have heard of Tuskegee and that’s just one…”
The Tuskegee Syphilis Study involved around 600 African American men, 400 of whom had syphilis, and was designed to observe the effects of the condition when left untreated. There is no evidence that participants gave their informed consent to taking part and those with syphilis were not informed of their diagnosis or offered available treatments. More than 100 men died as a result. The study was ultimately deemed unethical and the Office for Human Research Protections (OHRP) was created to oversee clinical trials in the US.
Another issue is that sites, particularly larger institutions, do not necessarily have details of diverse patient populations. With many sponsors preferring to approach these institutions to support clinical trial patient recruitment, Jane says this misses out a lot of smaller, community sites that are more likely to have a more diverse patient population. “That makes it a struggle when you’re trying to have representation,” Jane said.
Lack of site resource
From speaking to sites directly, we know that a lack of resource continues to be a big challenge. High turnover of study coordinators and other employees moved to other units in the healthcare system to provide support where needed. This puts extra pressure onto sites when they are trying to enroll patients for trials and particularly for those that have a lot of complexities.
Doctors acknowledging clinical trials as treatment options
There continues to be the challenge of doctors and physicians viewing clinical trials as viable treatment options. “…education is really needed, “ said Jane. “We can’t assume that doctors know about clinical trials and that they’re up on everything, or that they’re even telling their patients about clinical trials because unfortunately that doesn’t happen, again especially with patients of color.”
Cultural barriers to participating in decentralized trials
During the pandemic, we saw the accelerated adoption of decentralized clinical trials. While this model is helpful in reducing the burden of participation on a patient, decentralized trials may not be as convenient as some think. Home nursing, which is an important component of decentralized trials, is not always viewed favorably. According to Parexel, fewer than one in four (23 per cent) of patients are willing to have a nurse come to their home. “There’s a stigma in having home nursing come to you,” explained Jane. “Some people feel embarrassed about nurses coming to your home so there’s a lot of reasons why [it] doesn’t actually, in reality, work.” There is a real need to recognise when decentralized trials are appropriate and for whom. As Kate noted later in the webinar, more trials are adopting a hybrid model, bridging the gap between fully decentralized and site-based.
Accelerating clinical trial patient recruitment with a ‘boots-on-the-ground’ approach
Flexibility and time are both important when it comes to overcoming many clinical trial patient recruitment challenges. However, sites often struggle with both. To support them effectively and supercharge their clinical trial patient recruitment, Innovative Trials uses Clinical Enrollment Managers (CEMs) to provide on-the-ground, bespoke support. With more than 60 CEMs based in over 70 countries, each one has local, in-country expertise in healthcare and patient recruitment specifically. They work directly with sites to understand where they are in their recruitment journey and accelerate patient recruitment through individualized solutions. They provide both virtual and in-person support and all have extensive experience within the clinical trial arena. “It’s like your neighbor calling from just around the corner rather than someone calling from outside the country,” Whitney said. “We know that sites that feel well supported recruit extremely well compared to those that may be or are a little bit demotivated…so with the CEM support we’re able to engage with the sites and keep that momentum up to support their [patient recruitment] needs.”
For Parexel, using Innovative Trials’ CEMs to support sites has resulted in a 28 per cent higher screening rate and 38 per cent higher randomization compared with non-CEM supported sites across 12 studies. “The way CEMs function is that they really can provide so many different types of services that are tailormade to what the site needs,” Jane said. “…so it varies very much on a site-by-site, country-by-country level on what CEMs will do. That’s really the strength, I think, in what this brings to sites and to studies.”
Need help accelerating your clinical trial patient recruitment? Contact us about how our boots-on-the-ground approach promotes positive outcomes for patient recruitment and retention.