Black History Month
Innovative Trials are passionate about ensuring our diverse population is adequately represented within medical research. Whether it is wanting to see more people from underrepresented communities choosing science as a career and pushing for greater patient diversity in clinical trials or focusing on what we are doing internally to celebrate and promote equality and diversity.
Throughout 2021, we have made a pledge to share our education and experiences with our clients and colleagues, to ensure inclusivity across the board. Each month we will be releasing communications in line with national and international awareness campaigns.
So far in our Awareness series, we have explored: Women’s Health; diversity in Cancer, Heart Disease and Scleroderma, Ovarian and Prostate Cancer, Autism Acceptance,Ramadan and Bowel Cancer, Stroke and Hepatitis, Mental Health, Pride month, Women with Alopecia, Psoriasis, Dementia and Disability.
We will be looking into Black History Month as part of our first October Awareness blog.
Introduction

Black History Month recognises the great achievements and contributions of those with African or Caribbean heritage. It is also used as an opportunity to educate others about the impact of racism, underrepresentation of the black community and how to challenge negative stereotypes. [1] Innovative Trials are dedicated to working to ensure that improving diversity within clinical trials remains a priority. This Black History Month, we are discussing the racial disparities in clinical trials, the effects COVID-19 has had on the black community and how we can tackle this ongoing issue.
Black Representation in Clinical Trials
There is a compelling requirement for clinical trials to ensure there is an equitable representation of diverse racial and ethnic groups as opposed to the current over-representation of white individuals. [2]
There are several investigations that have revealed racial differences in drug response and indicate the need for adequate representation of racial minorities in clinical research and trials. However, there is concern that there is a disproportionate contribution of racial and ethnic minorities in clinical research. A study reviewed the representation of Black Americans in 50 recently published clinical trials of new drugs. It was revealed that investigators do not seem to consider racial differences as a potential source of variability. It was also found that in the majority of studies, the proportion of black subjects is less than their proportion in the general population. This underrepresentation in clinical trials suggests that not enough information exists to accurately assess the safety and efficacy of many new therapies in the black community. [3]
This work is crucial because unless the people who will be using a new therapy are represented in clinical trials, it is not possible to know how it will work for those who need it the most. For example, cardiovascular disease: Heart conditions disproportionately affect Black individuals, however a global trials report by the FDA, found that they only accounted for 2.5% of clinical trial participants. [4]
COVID-19
Coronavirus (COVID-19) has had a devastating impact on countries around the world. As we continue to battle this virus, there has been growing recognition that the pandemic has had a significant impact on black and minority ethnic communities. There is distinct evidence that black and minority ethnic groups are at higher risk of dying from COVID-19 than the rest of the population.[5] There are several factors that contribute to this including demographic and socio-economic factors as well as a patient’s previous health profile. After adjusting for age, a report compared the risk of COVID-19 related deaths across ethnic groups within the same socio-economic class.[6]
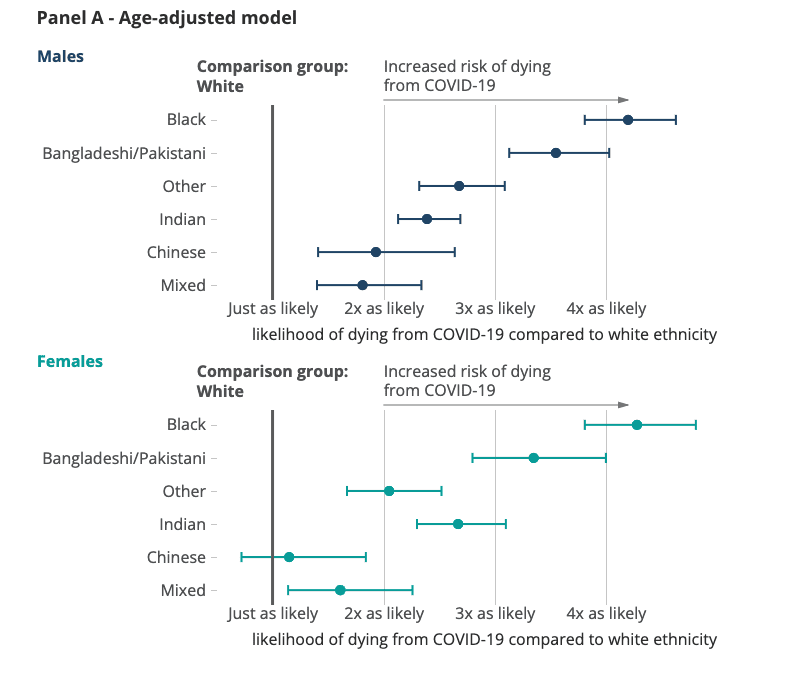
It was found that both black men and women were four times more likely to die from COVID-19 than the white comparison group.
Several studies have suggested that reasons for this include that the black and minority ethnic communities are more likely to have exposure to the virus as they often live in densely populated areas where the virus has spread fastest. Particularly in London, these individuals are also more likely to be key workers, increasing their chances of exposure to the virus. Some minority ethnic groups are more likely to live in overcrowded conditions, which increases the risk of transmission within households as well as the surrounding community. Additionally, many of the pre-existing health conditions that increase the risk of a more severe COVID infection also appear more commonly in minority and ethnic groups.
Despite this data, black and minority ethnic groups have not been adequately represented in clinical trials for the development of vaccines. Although people from these groups make up 13.8% of the UK population, only 5.7% of those participating in COVID-19 vaccination trials have been from ethnic minorities. [7]
What can be done?
In order to tackle this underrepresentation and reach the black community to ensure we see an increase in diversity within clinical trials moving forward, various approaches are necessary. It is important for research organisations to build trust within these minority communities by raising awareness amongst black communities, communicating with respected figures from these communities, answering any questions or concerns [7] and dismantling any negative preconceived notions that may exist about participating in clinical trials.
Innovative Trials and Diversity
Innovative Trials has launched a number of initiatives over the past couple of years to focus on recruiting patients from diverse populations. This includes diversity focussed mapping with sites: dedicated and directed questions to understand the sites abilities to recruit from diverse populations and also their previous experience in this area. Innovative Trials’ experienced Clinical Enrolment Managers (CEMs) can then work with sites to expand their potential and provide suggestions on recruiting patients that are truly representative of the population affected by the condition. – Leanne Keem, Business Unit Head.
The underrepresentation of black communities is one of the key aspects that led Innovative Trials to join efforts with Egality and COUCH Health and create The Road to Equality taskforce. Road to Equality is an initiative launched to improve diversity in UK health research and clinical trials. Finally, in an increasing number of studies Innovative Trials is taking on, boosting diversity in the patient population targeted and recruited has become one of the main KPIs to achieve. For example, in a Phase III Active Systemic Lupus Erythematosus (SLE) study Sites receiving diversity outreach visits saw a 63% increase in randomised patients than those without.
References
- [1] BBC News (October, 2020). Black History Month. Retrieved September 2021 from https://www.bbc.co.uk/news/explainers-54522248
- [2] D Ross Camidge, Haeseong Park, Karen E Smoyer, Ira Jacobs, Lauren J Lee, Zemfira Askerova, Justin McGinnis & Yousef Zakharia (2021). Race and ethnicity representation in clinical trials: findings from a literature review of Phase I oncology trials. Retrieved September 2021 from https://www.futuremedicine.com/doi/10.2217/fon-2020-1262
- [3] Craig K. Svensson, PharmD, PhD (1989). Representation of American Blacks in Clinical Trials of New Drugs. Retrieved September 2021 from https://jamanetwork.com/journals/jama/article-abstract/375922
- [4] Jocelyn Ashford (July, 2020). Clinical trials need to include more Black and other minority participants. Retrieved September 2021 from https://www.statnews.com/2020/07/22/clinical-trials-include-more-black-and-other-minority-participants/
- [5] Tim Elwell-Sutton, Sarah Deeny, Mai Stafford (May, 2020). Emerging findings on the impact of COVID-19 on black and minority ethnic people. Retrieved September 2021 from https://www.health.org.uk/news-and-comment/charts-and-infographics/emerging-findings-on-the-impact-of-covid-19-on-black-and-min
- [6] Office for National Statistics (May, 2020). Coronavirus (COVID-19) related deaths by ethnic group, England and Wales: 2 March 2020 to 10 April 2020. Retrieved September 2021 from https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronavirusrelateddeathsbyethnicgroupenglandandwales/2march2020to10april2020?hootPostID=229db5cd884a4f73d5bd4fadcd8959b
- [7] RACE.ED (May, 2021). Fixing the underrepresentation of BAME participants in medical research. Retrieved September 2021 from
https://www.race.ed.ac.uk/blog/2021/fixing-underrepresentation-bame-participants-medical-research
Equality, Diversity and Inclusion continues to be high on our agenda. We are working behind the scenes to push this forward. Keep your eyes peeled for more.
Find out how we’re working with clients to ensure greater patient diversity in clinical trials.
