
COVID-19
Providing solutions in the COVID-19 pandemic
Responding to the impact of Covid-19 on your clinical trials
Globally, patient recruitment into clinical trials has changed. The industry is starting to reopen sites and countries to enrolment, however due to the impact of the pandemic, many sites may require additional support to achieve enrolment targets.
How can Innovative Trials help?
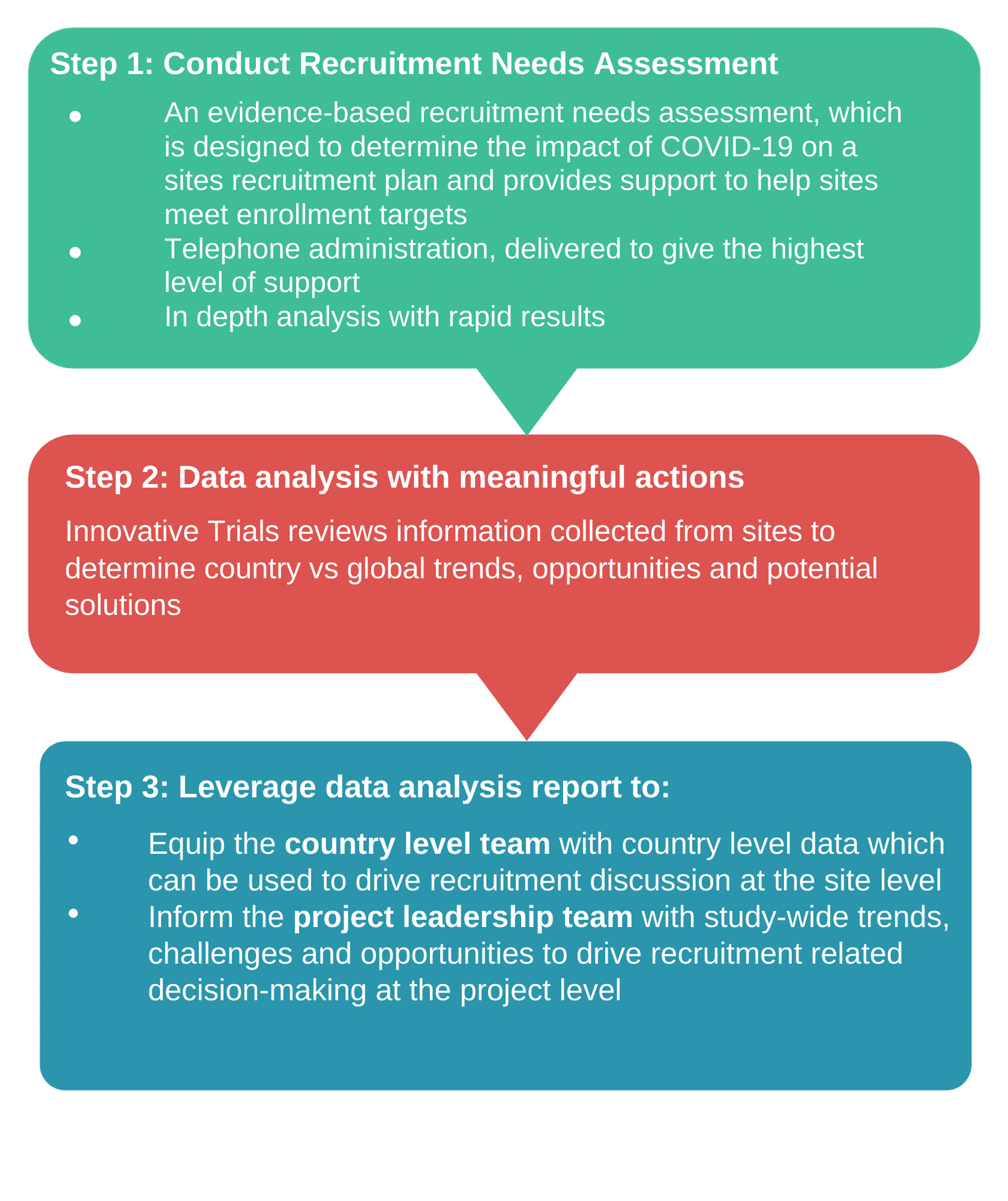
With over 10 years experience as a global provider of patient recruitment and retention services in over 50 countries globally, we have launched our Site Recruitment Needs Assessment. This new service has been developed to support the industry to establish a baseline of the site’s recruitment status, which takes into consideration how COVID-19 has impacted the patient recruitment funnel.
Once newly discovered recruitment constraints at the protocol, patient and operational level are assessed, your site and study team will be equipped with site, country and global level insights to further optimise enrolment for the project. A recommendations report will be provided to get you started, however we are also able to come along on the journey to support the implementation of the plan.
What’s included in the Recruitment Needs Assessment?
Please fill in the form below to receive more information and a bespoke estimate.
Are you conducting clinical trials with Covid-19 patients?
We have expertise in global patient recruitment for trials in acute care settings as well as recruiting healthy volunteers for vaccine studies. We offer a package of services to boost patient recruitment and reduce site burden to ensure successful study enrolment.
Click here to receive a bespoke estimate.
Complete the short questionnaire below to receive a bespoke estimate.
Bespoke Estimate
To help us provide you with an accurate response, please fill in this short questionnaire.
Your personal information is an important part of our service and keeping it safe and secure is our top priority. By completing and submitting our form you’re confirming you’re happy for us to securely store your personal details so we can get in touch whenever we have information that we think might be of interest to you. Read our Privacy Policy for more details about how we use and store data.
